Electroplating process with all variables
Electroplating is a widely used process in which a metal or alloy is deposited on a surface of a different material by means of an electrical current. This process has numerous applications in different fields such as electronics, automotive, aerospace, and jewelry making, among others. In this blog, we will discuss the electroplating process in detail, including the variables parameters and applications.
Electroplating Process: The electroplating process involves the deposition of a thin layer of metal on the surface of another material, typically a metal or plastic substrate. This is achieved by passing an electrical current through a solution containing dissolved metal ions and the substrate to be plated. The metal ions are reduced at the surface of the substrate, forming a thin layer of the desired metal.
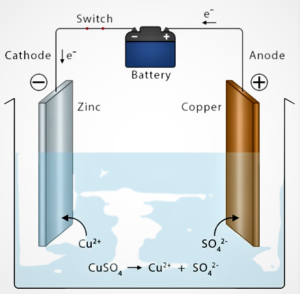
The electroplating process typically consists of the following steps:
Cleaning the substrate: The surface of the substrate is cleaned to remove any dirt, grease, or oxide layer that may interfere with the plating process.
Preparing the plating solution: The plating solution contains the dissolved metal ions and other additives that control the plating process, such as pH, temperature, and current density.
Plating the substrate: The substrate is immersed in the plating solution and connected to the negative electrode (cathode) of a DC power source. The metal to be deposited is connected to the positive electrode (anode) and dissolves into the solution as metal ions. The current passing through the solution causes the metal ions to be reduced at the surface of the substrate, forming a thin layer of the desired metal.
Post-treatment: After plating, the substrate may be treated to improve the adhesion, corrosion resistance, or other properties of the plated layer. This may involve rinsing, drying, heating, or applying a protective coating.
Variables Parameters: Several variables parameters affect the electroplating process, including:
Plating solution composition: The composition of the plating solution, including the concentration of metal ions, pH, temperature, and additives, affects the plating rate, quality, and uniformity.
Current density: The amount of current passing through the plating solution affects the rate and thickness of the plated layer, as well as the quality and uniformity.
Substrate material: The substrate material affects the adhesion, porosity, and compatibility of the plated layer. Different metals and plastics require different pre-treatment and plating conditions.
Plating time: The duration of the plating process affects the thickness and quality of the plated layer, as well as the cost and efficiency of the process.
Applications: The electroplating process has numerous applications in different fields, including:
Electronics: Electroplating is used to deposit thin layers of copper, nickel, gold, and other metals on printed circuit boards, connectors, and other electronic components.
Automotive: Electroplating is used to deposit thin layers of chrome, zinc, nickel, and other metals on automotive parts such as bumpers, wheels, and trim.
Aerospace: Electroplating is used to deposit thin layers of aluminum, titanium, and other metals on aircraft parts to improve their corrosion resistance, wear resistance, and conductivity.
Jewelry making: Electroplating is used to deposit thin layers of gold, silver, and other metals on jewelry to improve their appearance and durability.
Conclusion:
Electroplating is a widely used process in which a metal or alloy is deposited on a surface of a different material by means of an electrical current. This process has numerous applications in different fields, including electronics, automotive, aerospace, and jewelry making. The electroplating process involves several variables parameters, including the plating solution composition, current density, substrate material, and plating time, etc. Every variables affects the plating quality, property and its composition.